In this post we are going to share the detailed solution for class 10th science chapter 4. All the solutions are prepared by our esteemed teachers who are very well experienced in the teaching.
-
Complete Science Solutions Chapter Wise : Class 10th NCERT Science Solutions – All Chapters
Chapter 4 Solutions
EXERCISE :-1
QUE:-1 What would be the electron dot structure of carbon dioxide which has the formula CO2?
ANS:-
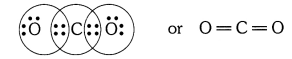
QUE:-2 What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – The eight atoms of sulphur are joined together in the form of a ring.)
ANS:-
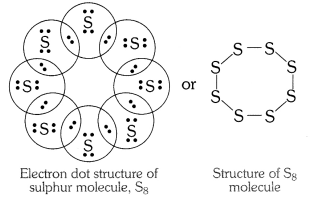
EXERCISE :-2
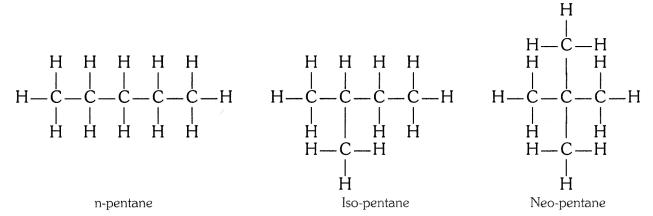
QUE:-1 How many structural isomers can you draw for pentane?
ANS:-
Three, these are n-pentane, iso-pentane and neo-pentane.
QUE:-2 What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
ANS: (i) Tetravalency
(ii) Catenation.
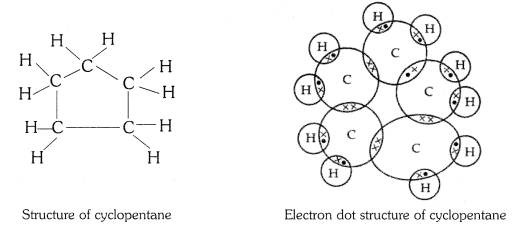
QUE:-3 What will be the formula and electron dot structure of cyclopentane?
ANS:-
The molecular formula of cyclopentane is C5 H10 .
The electron dot structure of cyclopentane is given
QUE:-4 Draw the structures for the following compounds.
(i) Ethanoic acid (ii) Bromopentane*
(iii) Butanone (iv) Hexanal.
*Are structural isomers possible for bromopentane?
ANS:-
(i) Ethanoic acid (CH3COOH)
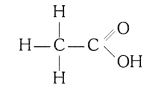
(ii) Bromopentane (C5H11Br)
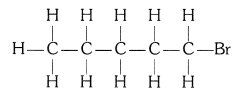
(iii) Butanone (CH3 — CH2 — COCH3)
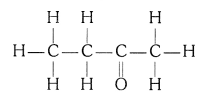
(iv) Hexanal (C5H11CHO)
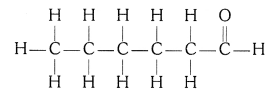
QUE:-5 How would you name the following compounds ?
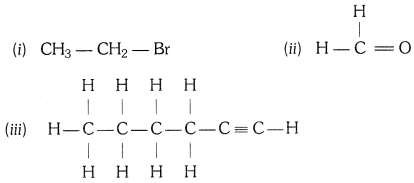
ANS:-
(i) Bromoethane
(ii) Methanal
(iii) 1 – Hexyne
EXERCISE :-3
QUE:- 1. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
ANS:-
Conversion of ethanol into ethanoic acid is an oxidation reaction because addition of oxygen to a substance is called oxidation. Here, oxygen is added to ethanol by oxidising agent like alkaline potassium permanganate or acidified potassium dichromate and it is converted into acid.

QUE:-2 A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
ANS:-
A mixture of ethyne and air is not used for welding because burning of ethyne in air produces a sooty flame due to incomplete combustion, which is not enough to melt metals for welding.
EXERCISE :-4
QUE:- 1. How would you distinguish experimentally between an alcohol and a carboxylic acid?
ANS:-
Differences between alcohol and carboxylic acid
| Test | Alcohol | Carboxylic acid |
| (i) Litmus test | No change in colour. | Blue litmus solution turns red. |
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | On heating, pink colour disappears. | Does not happen so. |
QUE:- 2 What are oxidising agents?
ANS:-
Oxidising agents are the substances which give oxygen to another substances or which remove hydrogen from a substance.
For example, acidic K2Cr2O7 is an oxidising agent, that converts (oxidises) ethanol into ethanoic acid.
EXERCISE :-5
QUE:- 1 Would you be able to check if water is hard by using a detergent?
ANS:- No, because detergents can lather well even in hard water. They do not form insoluble calcium or magnesium salts (scum). On reacting with the calcium ions and magnesium ions present in the hard water.
QUE:- 2 People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
ANS:-
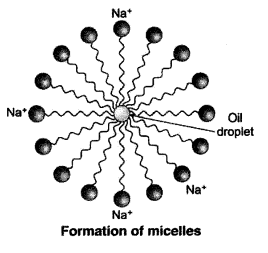
It is necessary to agitate to get clean clothes because the soap micelles which entrap oily or greasy particles on the surface of dirty cloth have to be removed from its surface. When the cloth wetted in soap solution is agitated or beaten, the micelles containing oily or greasy dirt get removed from the surface of dirty cloth and go into water and the dirty cloth gets cleaned.
EXERCISE :-6 ( chapter end question)
QUE:- 1 Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
ANS:-
(b) 7 covalent bonds.
QUE:- 2 Butanone is a four-carbon compound with the functional group
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
ANS:-
(c) Ketone.
QUE:- 3 While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
ANS:-
(b) The fuel is not burning completely.
QUE:- 4 Explain the nature of the covalent bond using the bond formation in CH3Cl.
ANS:-
Covalent bond is formed by sharing of electrons so that the combining atoms complete their outermost shell.
In CH3Cl : C = 6, H = 1 and Cl = 17 And their electronic configuration is C – 2,4, H – 1 and Cl – 2, 8, 7
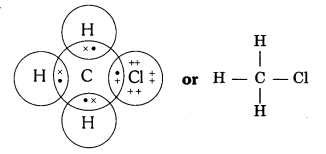
Three hydrogen atoms complete their shells by sharing three electrons (one electron each) of carbon atom.
Chlorine completes its outer shell by sharing its one out of seven electrons with one electron of carbon atom.
Thus carbon atom shares all its four electrons with three hydrogen atoms and one of chlorine atom and completes its outermost shell and single covalent bonds are formed in CH3Cl.
QUE:- 5 Draw the electron dot structures for
(a) ethanoic acid.
(b) H2S.
(c) propanone.
(d) F2 .
ANS:-
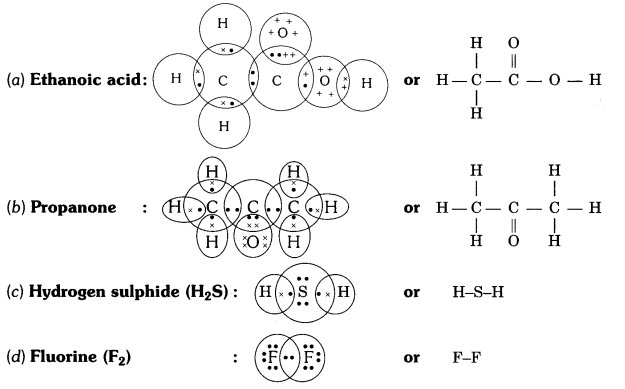
QUE:- 6 What is an homologous series? Explain with an example.
ANS:-
Homologous series : A homologous series is a group of organic compounds having similar structures and similar chemical properties in which the successive compounds differ by -CH2 group.
Characteristics of homologous series :
(i) All members of a homologous series can be represented by the same general formula. For example, the general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes number of carbon and hydrogen atoms in one molecule of alkane.
(ii) Any two adjacent homologues differ by one carbon atom and two hydrogen atoms in their molecular formulae.
(iii) The difference in the molecular masses of any two adjacent homologues is 14u.
(iv) All the compounds of a homologous series show similar chemical properties.
(v) The members of a homologous series show a gradual change in their physical properties with increase in molecular mass.
For example, general formula of the homologous series of alkanes is CnH2n+2, in which ‘n’ denotes number of carbon atoms in one molecule of alkane. Following are the first five members of the homologous series of alkanes (general formula CnH2n+2).
| Value of n | Molecular formula | Name of compound |
| 1 | CH4 | Methane |
| 2 | C2H6 | Ethane |
| 3 | C3H8 | Propane |
| 4 | C4H10 | Butane |
| 5 | C5H12 | Pentane |
QUE:- 7 How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
ANS:-
Difference on the basis of physical properties
| Property | Ethanol | Ethanoic acid |
| (i) State | Liquid | Liquid |
| (ii) Odour | Sweet smell | Pungent vinegar-like smell |
| (iii) Melting point | 156 K | 290 K |
| (iv) Boiling point | 351 K | 391 K |
Difference on the basis of chemical properties
| Test | Ethanol | Ethanoic acid |
| (i) Litmus test | No change in the colour of litmus solution. | Blue litmus solution turns red. |
| (ii) Sodium hydrogen carbonate test | C2H5OH + NaHCO3 → No reaction No brisk effervescence. | CH3COOH + NaHCO3 → CH3COONa + H2O + CO2 Brisk effervescence due to evolution of CO2. |
| (iii) Alkaline potassium permanganate | On heating, pink colour disappears. | Does not happen so. |
QUE:- 8 Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
ANS:-
Micelle formation takes place when soap is added to water because the hydrocarbon chains of soap molecules are hydrophobic (water repelling) which are insoluble in water, but the ionic ends of soap molecules are hydrophilic (water attracting) and hence soluble in water.
Such micelle formation will not be possible in other solvents like ethanol in which sodium salt of fatty acids do not dissolve.
QUE:- 9 Why are carbon and its compounds used as fuels for most applications?
ANS:- Carbon and its compounds give a large amount of heat per unit weight and are therefore, used as fuels for most applications.
QUE:- 10 Explain the formation of scum when hard water is treated with soap.
ANS:-Hard water contains salts of calcium and magnesium. Calcium and magnesium on reacting with soap form insoluble precipitate called scum. The scum formation lessens the cleansing property of soaps in hard water.
QUE:- 11 What change will you observe if you test soap with litmus paper (red and blue)?
ANS:-Red litmus will turn blue because soap is alkaline in nature. Blue litmus remains blue in soap solution.
QUE:- 12 What is hydrogenation? What is its industrial application?
ANS:- The addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydro-carbon is called hydrogenation. The process of hydrogenation takes place in the presence of nickel (Ni) or palladium (Pd) metals as catalyst.
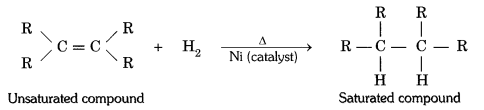
Application : The process of hydrogenation has an important industrial application. It is used to prepare vegetable ghee (or vanaspati ghee) from vegetable oils.
QUE:- 13 Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4.
ANS:- Addition reactions take place only in unsaturated hydrocarbons. So addition reaction take place only in C3H6 and C2H2.
QUE:- 14 Give a test that can be used to differentiate chemically between butter and cooking oil.
ANS:-
Butter is a saturated carbon compound while cooking oil is an unsaturated carbon compound. An unsaturated compound decolourises bromine water, while a saturated compound cannot decolourise it. So we can distinguish chemically between a cooking oil and butter by the bromine water. Add bromine water to a little of cooking oil and butter taken in separate test-tubes.
*Cooking oil decolourises bromine water showing that it is an unsaturated compound.
*Butter does not decolourise bromine water showing that it is a saturated compound .
QUE:- 15 Explain the mechanism of the cleaning action of soaps.
ANS:-
When a dirty cloth is put in water containing dissolved soap, then the hydrocarbon end of the soap molecules in micelle attach to the oil or grease particles present on the surface of dirty cloth. In this way the soap micelle entraps the oily or greasy particles by using its hydrocarbon ends. The ionic ends of the soap molecules in the micelles, however, remain attached to water. When the dirty cloth is agitated in soap solution, the oily and greasy particles present on its surface and entrapped by soap micelles get dispersed in water due to which the soap water becomes dirty but the cloth gets cleaned. The cloth is cleaned thoroughly by rinsing in clean water a number of times.
summary of the chapter
* Carbon is a versatile element that forms the basis for all living organisms and many of the things we use.
* This large variety of compounds is formed by carbon because of its tetravalency and the property of catenation that it exhibits.
* Covalent bonds are formed by the sharing of electrons between two atoms so that both can achieve a completely filled outermost shell.
* Carbon forms covalent bonds with itself and other elements such as hydrogen, oxygen, sulphur, nitrogen and chlorine.
* Carbon also forms compounds containing double and triple bonds between carbon atoms. These carbon chains may be in the form of straight chains, branched chains or rings.
* The ability of carbon to form chains gives rise to a homologous series of compounds in which the same functional group is attached to carbon chains of different lengths.
*The functional groups such as alcohols, aldehydes, ketones and carboxylic acids bestow characteristic properties to the carbon compounds that contain them.
*Carbon and its compounds are some of our major sources of fuels.
* Ethanol and ethanoic acid are carbon compounds of importance in our daily lives.
* The action of soaps and detergents is based on the presence of both hydrophobic and hydrophilic groups in the molecule and this helps to emulsify the oily dirt and hence its removal.
You can see other posts here:
- Class 10th NCERT Science Chapter 1 – Chemical Reactions and Equations Solutions
- NCERT Class 10th Science Chapter 2- Acids, Bases and Salts Solutions
- NCERT Class 10th Science Chapter – 3 Metals and Non Metals
-
Complete Science Solutions Chapter Wise : Class 10th NCERT Science Solutions – All Chapters
Tags : Class 10th chapter 4 science solution, Science class 10th chapter 4 solution, NCERT science class 10 solution, Class 10th solution, Solution for class 10th chapter 4.
